ABSTRACT
In
countries like Madagascar, where biological diversity is high but also
highly threatened, it is imperative that we gather a baseline of information
on little known taxa. This information will have broad application for
future work in systematics and conservation. For this research, the tribe
Platynini was chosen because it is the most diverse and abundant of the
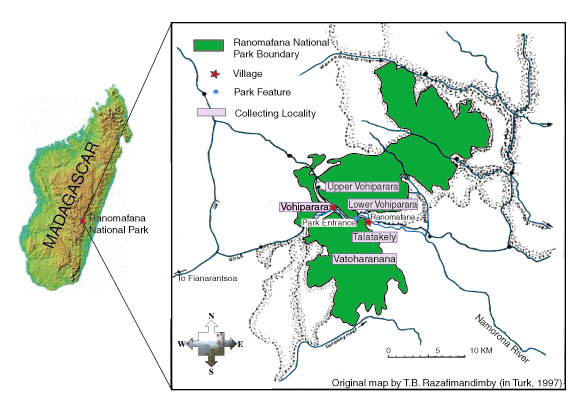
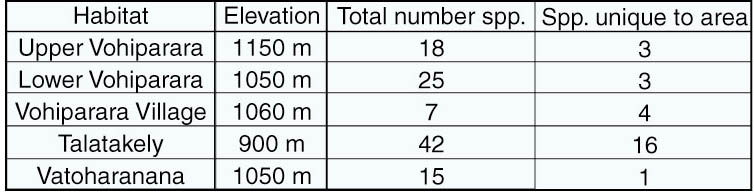
ground beetle tribes represented in Ranomafana National Park (RNP). Work
with previously collected specimens, and a collecting expedition to RNP,
have identified at least 56 morphospecies in 10 different genera. Almost
all of these are new locality records for RNP, including at least 18 undescribed
species. A species list for RNP, with all species compared to type specimens,
will be created. Microhabitat and collecting method information, known
distributions for each species within RNP and across Madagascar, and a
key to the species of RNP (both online and in standard form) will be included
in this research. We also discuss the apparent seasonality of the carabid
fauna at RNP, the difficulty and inappropriateness of standard mass collecting
techniques and quantitative sampling for carabids in tropical areas, and
the need for collaboration with local Malagasy people in order to conduct
a thorough inventory for this or any group.